Describe the Bohr Model of the Hydrogen Atom
The electrons of the atom revolve around the nucleus in definite circular paths known as orbits. In 1913 Niels Bohr proposed the atomic Hydrogen model.

Bohr Model Of The Atom Chemtalk
Bohrs model of the hydrogen atom proposed by Niels Bohr in 1913 was the first quantum model that correctly explained the hydrogen emission spectrum.
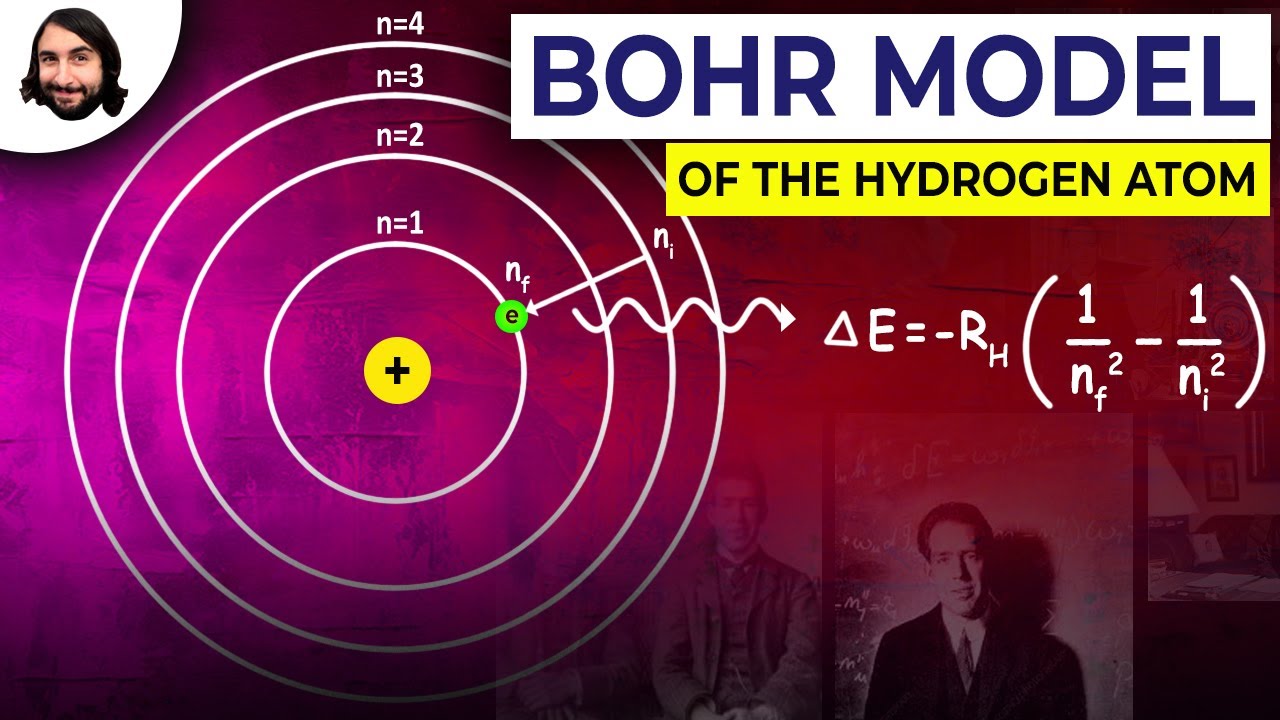
. E n frac 136rm eV n2 where n is the principal quantum. His first proposal is that only certain orbits are allowed. Bohr model of the hydrogen atom was the first atomic model to successfully explain the radiation spectra of atomic hydrogen.
Bohr Model of the hydrogen atom attempts to plug in certain gaps as suggested by Rutherfords model. Niels Bohr introduced the atomic hydrogen model in 1913. It has a special place in history because it introduced quantum theory which gave rise to quantum mechanics.
According to Bohrs theory. We say that the orbits of electrons in atoms are quantized. Bohr Model of Hydrogen The simplest example of the Bohr Model is for the hydrogen atom Z 1 or for a hydrogen-like ion Z 1 in which a negatively charged electron orbits a small positively charged nucleus.
Postulates of Bohrs model of the atom. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. This model would change the way we understand the atomic structure of hydrogen.
Laminiaduo7 and 6 more users found this answer helpful. It was preceded by the Rutherford nuclear model of the atom. 1 an electron moves around the nucleus in a circular orbit 2 an electrons angular momentum in orbit is quantized and 3 the change in an electrons energy as it makes a quantum jump from one orbit to another is always accompanied by the emission or absorption of a photon.
Key points Bohrs model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells or orbits around the nucleus. For hydrogen and hydrogen-like atoms the Bohr model of hydrogen gives the energy E of an electron present in the n energy level orbit of hydrogen as. It holds a special place in history as it gave rise to quantum.
Bohrs Model of Hydrogen Atom The first atomic model to successfully describe the radiation spectra of atomic hydrogen was Bohrs model of the hydrogen atom. The farther the electron is from the nucleus the the energy of the system. In 1913 Niels Bohr proposed the atomic Hydrogen model.
According to the Bohr model for the hydrogen atom the energy of the atom is not continuous but has certain discrete energy each of which is related to a fixed circular of the electron around the nucleus. BOHR MODEL OF THE HYDROGEN ATOM De Broglies Hypothesis de Broglies hypothesis that electrons have a wavelength λ hmv gave an explanation for Bohrs quantised orbits by bringing in the wave particle duality. Bohr was able to derive the formula for the hydrogen spectrum using basic physics the planetary model of the atom and some very important new proposals.
The Bohr Model of The Atom 1 The H atom only has certain energy levels they are determined by fixed circular orbits of electrons around the nucleus. Bohrs model of atom holds a distinct place in the past as it gave rise to quantum mechanics by proposing the quantum theory. The atom consists of a small positively charged nucleus at its centre and surrounded by negatively charged electrons in a definite circular path.
Bohr Model of Hydrogen Atom Quantised. The Bohr Model of the Hydrogen Atom tries to fill in some of the holes left by Rutherfords model. The Bohr Model of Hydrogen-like Atoms.
The model proposed in 1913 by the Danish physicist Niels Bohr and later further developed by Arnold Sommerfeld to describe the hydrogen spectrum was of great importance in the historical development of atomic theory. Bohr model of the hydrogen atom was the first atomic model to effectively describe the radiation spectra of atomic hydrogen. The Bohr Model of the Hydrogen Atom tries to fill in some of the holes left by Rutherfords model.
The orbits correspond to circular standing waves in which the circumference of the orbit equals a whole number of wavelength. We say that the orbits of electrons in atoms are quantized. The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms.
He described it as a positively charged nucleus comprised of protons and neutrons surrounded by a negatively charged electron cloud. How Bohrs model of hydrogen explains atomic emission spectra. Bohr was able to derive the formula for the hydrogen spectrum using basic physics the planetary model of the atom and some very important new proposals.
In 1915 the Bohr Model also known as Bohr Atomic Model was proposed by a scientist named Niels Bohr. In Rutherfords model an atom consists of a positively charged point-like nucleus that contains almost the entire mass of the atom and of negative electrons that are located far. Bohrs model combines the classical mechanics of planetary motion with the quantum concept of photons.
See the answer Show transcribed image text Expert Answer 100 1 rating. Bohrs model of the hydrogen atom is based on three postulates. 2 An atoms energy does not change while the electron moves in a particular orbit.
Bohr model of hydrogen atom formula. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Even though it is very different from the modern description of an atom it is.
In the model electrons orbit the nucleus in atomic shells. Niels Bohr introduced the atomic Hydrogen model in the year 1913. He was able to use research that indicated hydrogen emission spectra to explain how electrons interacted with the nucleus of an atom.
He postulated that the electron was restricted to certain orbits characterized by discrete energies. His first proposal is that only certain orbits are allowed. Niel Bohr presented the atomic Hydrogen model in the year 1913.
The Bohr model of hydrogen was the first model of atomic structure to correctly explain the radiation spectra of atomic hydrogen. The nucleus contains all the protons and neutrons of the atom. Bohrs model calculated the following energies for an electron in.
Reset Help proton The Bohr model for hydrogen places the single electron in a circular orbit around the electron The orbit of the is That is it has a molecule energy at a distance from the neutron random quantized This problem has been solved.
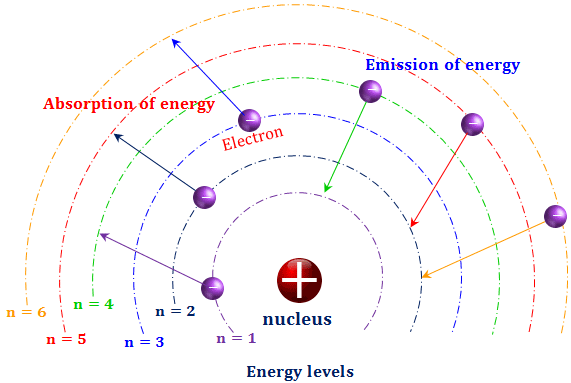
Bohr Model Hydrogen Atom Postulates Energy Levels
.PNG)
The Bohr Model Of The Hydrogen Atom

No comments for "Describe the Bohr Model of the Hydrogen Atom"
Post a Comment